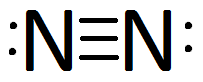Nitrogen is essential to life on earth. Nitrogen is the most common molecule in the earth's atmosphere. However the nitrogen in the earth's atmosphere is inaccessible to living organisms. The reason the nitrogen in the earth's atmosphere is inaccessible to living things is that the nitrogen molecule does not react easily with other molecules. A nitrogen molecule is made of two nitrogen atoms tied together by a triple bond. A triple bond is extremely hard to break. The process of freeing nitrogen atoms from the triple bond is called nitrogen fixation. Nitrogen that has been freed by nitrogen fixation is called fixed nitrogen. And fixed nitrogen is the nitrogen that all life uses. Nitrogen fixation can happen in nature. Usually it is done by certain anaerobic organisms or as a result of a lightning strike. Humans learned how to fix nitrogen in the twentieth century using a technique called the Haber-Bosch process.